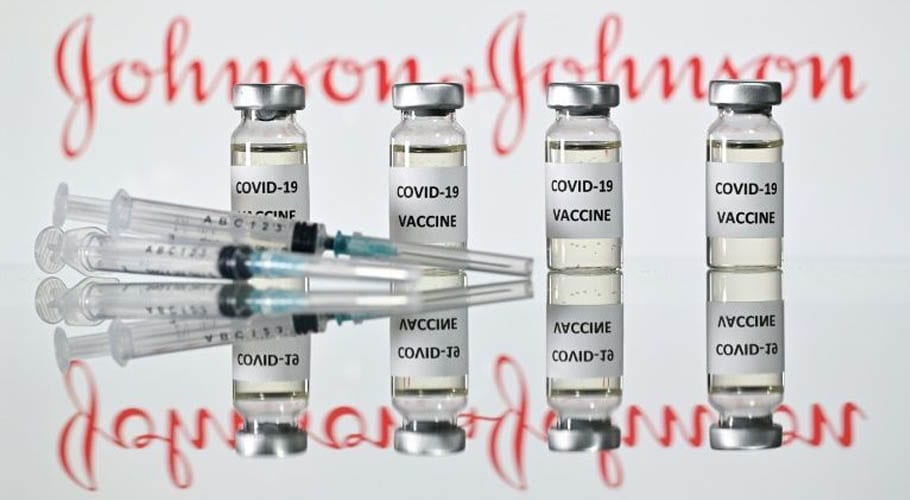
WASHINGTON: The United States authorized Johnson & Johnson’s Covid vaccine for emergency use, giving the nation a third shot to battle the outbreak.
The single-shot vaccine is highly effective in preventing severe COVID-19, including against newer variants, the Food and Drug Administration (FDA) said before giving the approval.
“This is exciting news for all Americans, and an encouraging development in our efforts to bring an end to the crisis,” US President Joe Biden said in a statement. He urged Americans to remain vigilant with anti-virus curbs such as social distancing, warning that new variants of the virus still posed a threat.
A third vaccine is seen as a vital means to ramp up the immunization rate in the United States, where more than 500,000 people have lost their lives to the coronavirus. In large clinical trials, the J&J vaccine’s efficacy against severe disease was 85.9 percent in the United States, 81.7 percent in South Africa, and 87.6 percent in Brazil.
Overall, among 39,321 participants across all regions, the efficacy against severe COVID-19 was 85.4 percent, but it fell to 66.1 percent when including moderate forms of the disease. Crucially, analyses of various demographic groups revealed no marked differences across age, race, or people with underlying conditions.
The J&J vaccine is the third to be approved in the United States after Pfizer’s and Moderna’s were provisionally approved in December. Over 65 million people in America have so far received at least one shot of either the Pfizer or Moderna vaccines but unlike those, the J&J vaccine requires just one dose, and is stored at fridge temperatures, offering logistical and practical advantages.
The J&J shot appears less protective than Pfizer and Moderna’s two-shot regimens, which both have an efficacy of around 95 percent against all forms of Covid-19 from the classic coronavirus strain. All three have been shown to fully protect against hospitalizations and death.
The company has announced it aims to deliver 20 million doses by the end of March, with 100 million by June though the US is pushing to expedite that timeline. The J&J vaccine uses a common-cold causing adenovirus, which has been genetically modified so that it can’t replicate, to carry the gene for a key protein of the coronavirus into human cells.