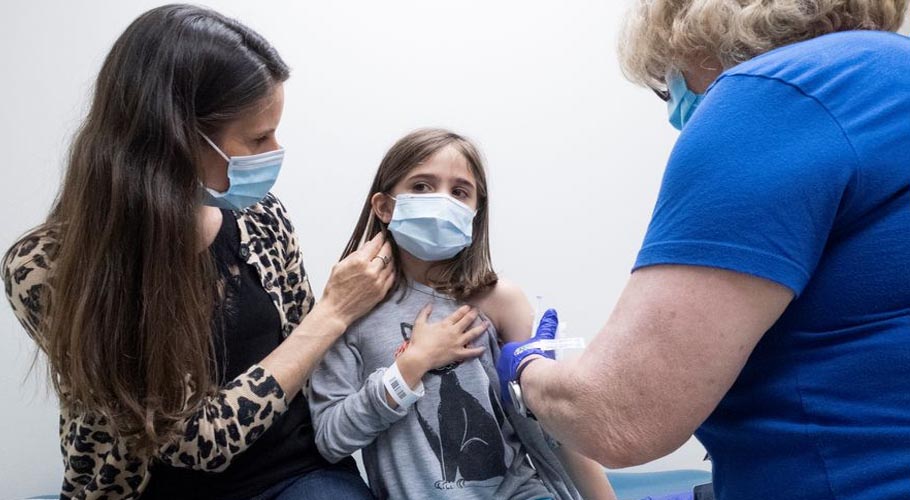
WASHINGTON: The United States Food and Drug Administration (FDA) has allowed the use of the Pfizer-BioNTech coronavirus vaccine for children aged 12 to 15 years old.
This is the first coronavirus vaccine to be authorized in the US for ages 12 to 15 years old. Vaccinating younger ages is considered a vital pace for getting children back into schools safely. U.S. President Joe Biden has called states to make the vaccine accessible to younger adolescents as soon as possible.
President Biden issued a statement welcoming the authorization as “a promising development in the country’s fight against the COVID-19 pandemic.
“If you are a parent who wants to guard your child, or a youngster who is interested in getting vaccinated, today’s decision is a step closer to that goal,” Biden added.
Meanwhile, Commissioner Janet Woodcock in a statement said, “Today´s action permits for a younger population to be guarded from the virus, bringing us nearer to returning to a sense of normality and to ending the plague,”
Woodcock further said, “Parents and guardians can rest confident that the agency commenced a hard and thorough review of all available statistics, as we have with all of our coronavirus vaccine emergency use approvals.”
The Food and Drug Administration previously allowed an emergency use permission for the Pfizer-BioNTech vaccine to individuals aged 16 and older.
Peter Marks, director of the FDA´s Center for Biologics Evaluation and Research said, “Having a vaccine approved for a younger population is an important step in continuing to diminish the huge public health burden caused by the coronavirus plague.”
According to the FDA, approximately 1.5 million coronavirus cases in individuals aged 11 to 17 years old have been reported to the US Centers for Disease Control and Prevention between March 1, 2020 and April 30, 2021.
The course of the ailment is usually milder in children but they can pass it on to older, more susceptible adults. In March 2021, Pfizer and its partner BioNTech said that their two-dose vaccine regimen was shown to be safe and highly effective in a trial of 2,260 12 to 15-year-olds.
Last week, President Joe Biden stressed the significance of expanding vaccinations to 12 to 15-year-olds and said the authorities were “ready to move immediately” once the authorization came through.
Some 20,000 pharmacies around the country were ready to begin to vaccinate adolescents, he said, and doses will also be shipped to paediatricians.
Covid-19 vaccines from Moderna and Johnson & Johnson have also received emergency use authorizations from the FDA but only for individuals over the age of 18.
About 46 percent of people in the US have received at least one shot of a coronavirus vaccine, according to statistics issued by the U.S. Centers for Disease Control and Prevention (CDC).
However, the pace of U.S. vaccinations has slowed significantly since peaking at a seven-day average of more than 3.3 million doses a day in mid-April. That average had fallen by more than a third to around 2.1 million shots a day as of May 4, as per the CDC data.